What Are Biologics for Severe Asthma?
When standard asthma treatments like inhalers and steroids stop working, biologics offer a different kind of help. These aren’t regular pills or sprays. They’re lab-made antibodies - proteins designed to zero in on specific parts of your immune system that fuel severe asthma. Think of them as precision missiles instead of scatter shots.
Two major types of biologics are used for severe asthma: anti-IgE and anti-IL-5. Omalizumab (Xolair) is the oldest and most well-known anti-IgE drug. It blocks immunoglobulin E, the antibody that triggers allergic reactions. Anti-IL-5 drugs - like mepolizumab (Nucala), reslizumab (Cinqair), and benralizumab (Fasenra) - target interleukin-5, a molecule that tells eosinophils (a type of white blood cell) to swarm the lungs and cause inflammation.
These drugs don’t cure asthma. But for people who keep ending up in the ER despite using their inhalers correctly, they can be life-changing. Clinical trials show they cut severe flare-ups by 40% to 60%. That means fewer hospital stays, less need for oral steroids like prednisone, and better daily breathing.
How Anti-IgE Works: Omalizumab Explained
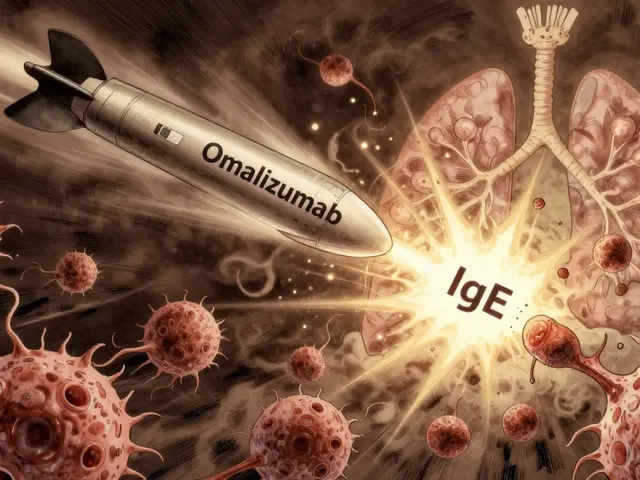
Omalizumab is the only biologic approved for allergic asthma. It’s for people whose asthma is clearly tied to allergies - like dust mites, pet dander, or mold. To qualify, you need a positive allergy test and a blood IgE level between 30 and 1500 IU/mL.
Here’s how it works: IgE normally sticks to mast cells and basophils in your airways. When you breathe in an allergen, those cells explode, releasing histamine and other chemicals that cause wheezing and tightness. Omalizumab binds to IgE before it can attach to those cells. No IgE binding = no explosion = less inflammation.
It’s given as a shot under the skin every 2 to 4 weeks, depending on your weight and IgE level. Many patients notice improvement after 3 to 4 months. Some feel better sooner. Others need longer. The INNOVATE trial showed a 50% drop in asthma attacks in people who used it correctly.
But omalizumab doesn’t work if your asthma isn’t allergic. If your eosinophil count is high but your IgE is normal, this drug won’t help. That’s why testing matters.
How Anti-IL-5 Drugs Fight Eosinophilic Asthma
If your asthma is driven by too many eosinophils - not allergies - then anti-IL-5 drugs are your best bet. These include mepolizumab, reslizumab, and benralizumab. All three lower eosinophil levels, but they do it differently.
Mepolizumab and reslizumab lock onto IL-5 itself, stopping it from telling eosinophils to multiply. Reslizumab is the only one given by IV infusion every 4 weeks. The rest are self-injected.
Benralizumab is the most aggressive. It doesn’t just block IL-5 - it attaches to the IL-5 receptor on eosinophils and signals your immune system to kill them outright. Within 24 hours, blood eosinophil counts can drop by 95%. That’s faster than any other biologic.
To qualify, you need a blood eosinophil count of at least 150 cells/μL in the past year - or 300 cells/μL if you’ve had recent flare-ups. The MENSA and ZONDA trials showed 50%+ reductions in exacerbations. Patients on benralizumab also cut their oral steroid use by nearly half.

Who Gets These Drugs - and Who Doesn’t
Not everyone with severe asthma qualifies. Biologics are only for people who’ve tried everything else: high-dose inhaled steroids, long-acting beta agonists, and sometimes oral steroids - and still struggle.
Doctors don’t just guess. They test. Blood eosinophils. Total IgE. FeNO (fractional exhaled nitric oxide). Asthma control scores. Adherence checks. Did you forget your inhaler? Are you using it wrong? That has to be ruled out first.
Real-world data shows 30% to 40% of people who start a biologic don’t respond. Why? Because they didn’t match the right drug to their asthma type. Giving omalizumab to someone with low IgE and high eosinophils? It won’t work. Giving mepolizumab to someone with allergic asthma but normal eosinophils? Also useless.
That’s why biomarkers are everything. You need the right test before the right drug.
Side Effects and Risks
Biologics are generally safe, but they’re not risk-free. The most common side effects? Soreness at the injection site, headache, and sore throat. These happen in up to 1 in 10 people and usually fade after a few doses.
More serious reactions are rare. Anaphylaxis - a sudden, life-threatening allergic reaction - happens in about 1 in 1,000 injections. It’s slightly higher (1 in 100) if you’ve had severe allergies before. That’s why the first few doses are given in a clinic, where staff can respond quickly.
Some patients report joint pain, fatigue, or muscle aches. One Reddit user stopped benralizumab after three doses because of severe joint pain, even though their asthma improved. Another reported zero ER visits after six months on mepolizumab and stopped daily prednisone.
Long-term safety data beyond five years is still being gathered. So far, no major red flags have emerged.
Cost, Access, and Real-World Challenges
These drugs cost between $25,000 and $40,000 a year in the U.S. Insurance rarely covers them without prior authorization - and that process can take 2 to 3 weeks. Many patients wait months before starting.
Even when approved, affordability is a barrier. Co-pay assistance programs from manufacturers help, but they don’t fix the bigger problem: access. Only 1% to 2% of eligible patients in the U.S. and Europe are on biologics. In Asia, it’s even lower.
Self-injection training is usually offered. Most people get comfortable after two or three supervised shots. But the cost and frequency of visits still weigh on patients. Some switch to a less effective inhaler just to avoid the hassle.

What’s Next for Biologics?
The future is getting brighter. Tezepelumab (Tezspire), approved in 2021, works differently. It blocks TSLP, an early warning signal from the airway lining. It helps even if you don’t have high eosinophils or IgE - meaning it could work for more people.
Now, trials are testing twice-yearly injections. That would cut the number of visits from 12 to 2 per year. Also, new AI tools are being built to predict who will respond to which drug, based on blood tests, history, and genetics.
And yes - the market is growing fast. Global sales hit $5.2 billion in 2023. Analysts predict they’ll top $12 billion by 2028. But growth depends on one thing: making these drugs easier to get, not just more powerful.
Is a Biologic Right for You?
If you’re still struggling with asthma despite using your inhalers correctly, talk to your specialist. Ask for:
- A blood test for eosinophils
- A serum IgE test
- A FeNO test
- A review of your asthma control score
- A check on your inhaler technique
If your numbers point to allergic asthma (high IgE), omalizumab might be your path. If your numbers show high eosinophils, anti-IL-5 drugs are the way to go.
Don’t assume it’s too expensive or too complicated. Many patients get financial help. Many learn to self-inject without trouble. And for those who respond? The difference isn’t just clinical - it’s personal. Fewer nights awake. No more emergency rides. Freedom to breathe without fear.
Final Thoughts
Biologics aren’t magic. They’re not for everyone. But for the right person - the one who’s tried everything and still can’t catch their breath - they’re the most powerful tool we have. They turn severe asthma from a daily battle into a manageable condition. And that’s worth fighting for.





Bradford Beardall
I've seen patients on omalizumab go from 3 ER visits a year to zero. It's not magic, but it's close. The real win is ditching prednisone. No more moon face, no more insomnia, no more crushing fatigue. Just breathing.
And the self-injection? Easier than people think. First time is nerve-wracking, second time is fine, third time you're doing it while scrolling TikTok.
Christine Milne
The notion that these drugs are somehow 'revolutionary' is a marketing fantasy perpetuated by pharmaceutical executives and compliant clinicians. The data is statistically significant but clinically marginal for most. And the cost? Absurd. In a country where insulin is rationed, we're spending $40,000 annually on antibody infusions for a condition that should be managed with better air quality and smoking cessation policies.
McCarthy Halverson
Test before you treat. Eosinophils. IgE. FeNO. If you skip these, you're guessing. And guessing kills. I've seen three patients on the wrong biologic. All wasted time. All wasted money. All still in the ER.
Do the test. Then act.
Michael Marchio
You people are missing the forest for the trees. Biologics are not the solution-they're the symptom of a broken healthcare system that rewards complexity over simplicity. Why are we injecting expensive proteins into people when we could be fixing the air they breathe? The EPA has known for decades that urban pollution worsens asthma. But no one wants to regulate Big Oil or Big Auto. So instead, we give people $40,000 shots to compensate for corporate negligence. It's not medicine. It's corporate damage control dressed in white coats.
Jake Kelly
I work with a lot of teens who have severe asthma. One of them started on benralizumab last year. She went from missing 20 days of school to zero. She joined the track team. She slept through the night for the first time since she was 8. That’s not a statistic. That’s a life.
Yes, it’s expensive. But some things are worth the cost.
Ashlee Montgomery
The real question isn't whether the drugs work-it's why we wait until people are broken before we offer them. Why isn't this part of routine care for moderate-severe asthma? Why is it a last resort? We treat cancer earlier. We treat diabetes earlier. But asthma? Wait until the lungs are scarred and the steroids have wrecked the bones.
Maybe we're treating the wrong thing.
neeraj maor
Did you know the FDA approved these drugs after only 6-month trials? The long-term data? Still being gathered. Meanwhile, the same companies that make these drugs also fund the asthma associations that push them. And the patents? Designed to last 20 years-so they can keep prices high. You think this is science? It's capitalism with a stethoscope. And the eosinophil count? Easily manipulated by stress, sleep, even the moon phase. Don't trust the numbers. Trust your gut.
Bradford Beardall
I get where you're coming from, but I’ve seen the opposite too. A guy on omalizumab who couldn’t walk to his mailbox without wheezing? Now he hikes every weekend. His wife said he finally started laughing again. That’s not just data. That’s human. Maybe the system’s broken, but this still fixes something real.