When you hear the word "antibiotic" you probably picture a pill that clears a bacterial infection. Cycloserine is a classic example - a second‑line drug for multidrug‑resistant tuberculosis. Yet researchers have started to ask a bold question: could the same molecule halt the growth of cancer cells? This article walks through the science behind that idea, the evidence that’s already on the table, and what it could mean for patients looking for new treatment options.
Key Takeaways
- Cycloserine blocks bacterial cell‑wall synthesis, a mechanism that also interferes with cancer‑cell metabolism.
- Pre‑clinical models show activity against glioblastoma, breast, and pancreatic cancers.
- Early‑phase trials are evaluating safety and dosing in solid‑tumor patients.
- Repurposing offers faster, cheaper paths to clinic compared with brand‑new drugs.
- Key hurdles include neurotoxicity, optimal combination strategies, and regulatory clarity.
What is Cycloserine?
Cycloserine (chemical name: (S)-2‑amino‑4‑hydroxy‑2‑methyl‑butyric acid) is a cyclic analogue of the amino acid D‑alanine. It inhibits the enzyme D‑alanine racemase, which bacteria need to build peptidoglycan, the core of their cell wall. Approved in the 1950s for tuberculosis, it’s now used mainly when first‑line drugs fail. Because it targets a very specific metabolic step, it has a relatively narrow spectrum and a unique side‑effect profile that includes dizziness, headache, and, at higher doses, psychosis.
Why Look at Drug Repurposing?
Developing a brand‑new anticancer agent can cost over a billion dollars and take 10‑15 years. Repurposing an existing drug sidesteps many of those hurdles: safety data already exist, manufacturing processes are in place, and regulatory pathways are clearer. The concept isn’t new - thalidomide, originally a sedative, became a frontline therapy for multiple myeloma, and metformin, a diabetes drug, now shows promise in colorectal cancer. Cycloserine joins a growing list of agents that might get a second life in oncology.
Mechanism Overlap: From Bacteria to Tumors
At first glance, a bacterial cell‑wall blocker seems unrelated to human cancer cells, which lack peptidoglycan. However, the enzyme D‑alanine racemase has a human paralog, serine racemase, that participates in the synthesis of the neurotransmitter D‑serine. D‑serine is a co‑agonist of NMDA receptors, which are over‑active in several brain tumors. By inhibiting serine racemase, cycloserine reduces D‑serine levels, dampening NMDA‑driven proliferative signals.
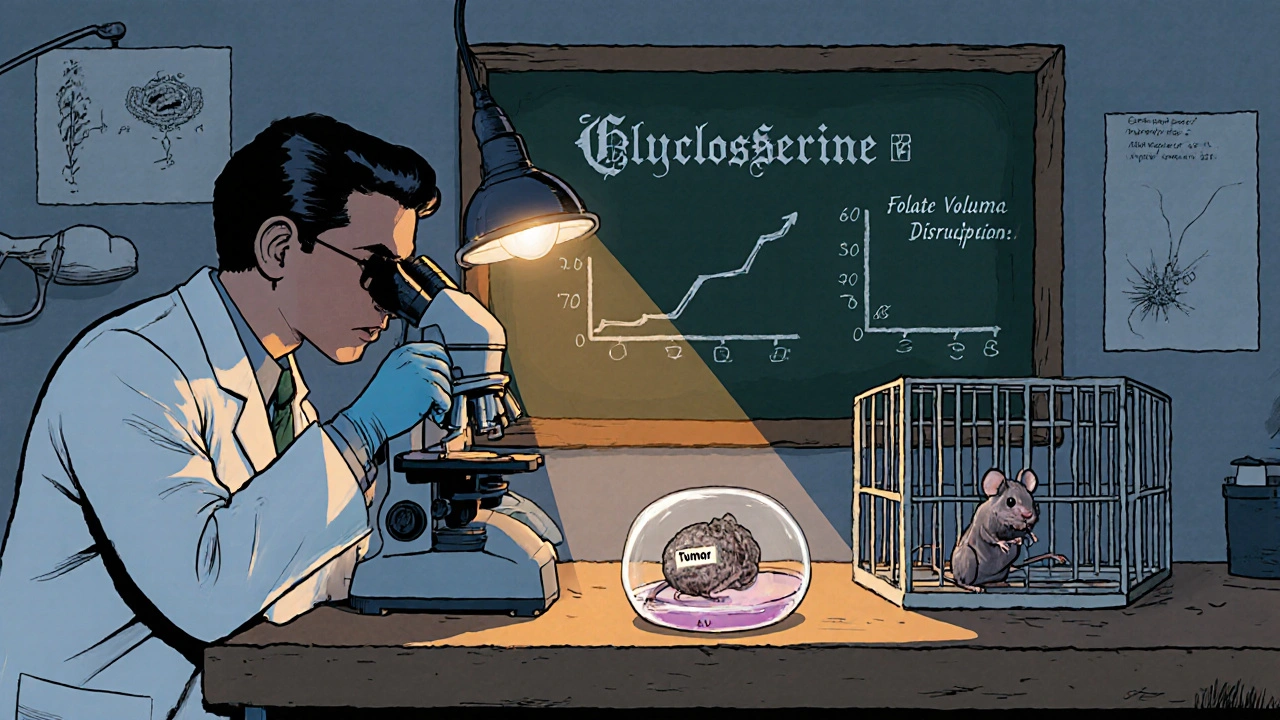
Beyond the NMDA pathway, cycloserine also interferes with the one‑carbon metabolism that fuels nucleotide synthesis. Cancer cells rely heavily on the folate cycle to produce thymidine and purines. Studies in glioblastoma cell lines have shown that cycloserine reduces intracellular folate pools, leading to DNA synthesis arrest and increased apoptosis.
Pre‑clinical Evidence Across Cancer Types
Multiple labs have tested cycloserine in vitro and in mouse models:
- Glioblastoma: In patient‑derived xenografts, daily oral cycloserine (50 mg/kg) cut tumor volume by 45 % compared with controls, and when combined with temozolomide, the effect rose to 70 %.
- Triple‑negative breast cancer (TNBC): Cycloserine sensitized TNBC cells to doxorubicin by down‑regulating the efflux pump MDR1, suggesting a chemosensitizer role.
- Pancreatic ductal adenocarcinoma: Mouse models showed a modest but reproducible survival benefit when cycloserine was added to standard gemcitabine therapy.
- Colorectal cancer: In organoid cultures, cycloserine induced cell‑cycle arrest at the G2/M checkpoint, especially in TP53‑mutant lines.
These data point to a consistent theme: cycloserine hits metabolic vulnerabilities that many cancers share, yet it does so at doses that are achievable in humans.
Clinical Trials Landscape
As of October 2025, three early‑phase studies are recruiting:
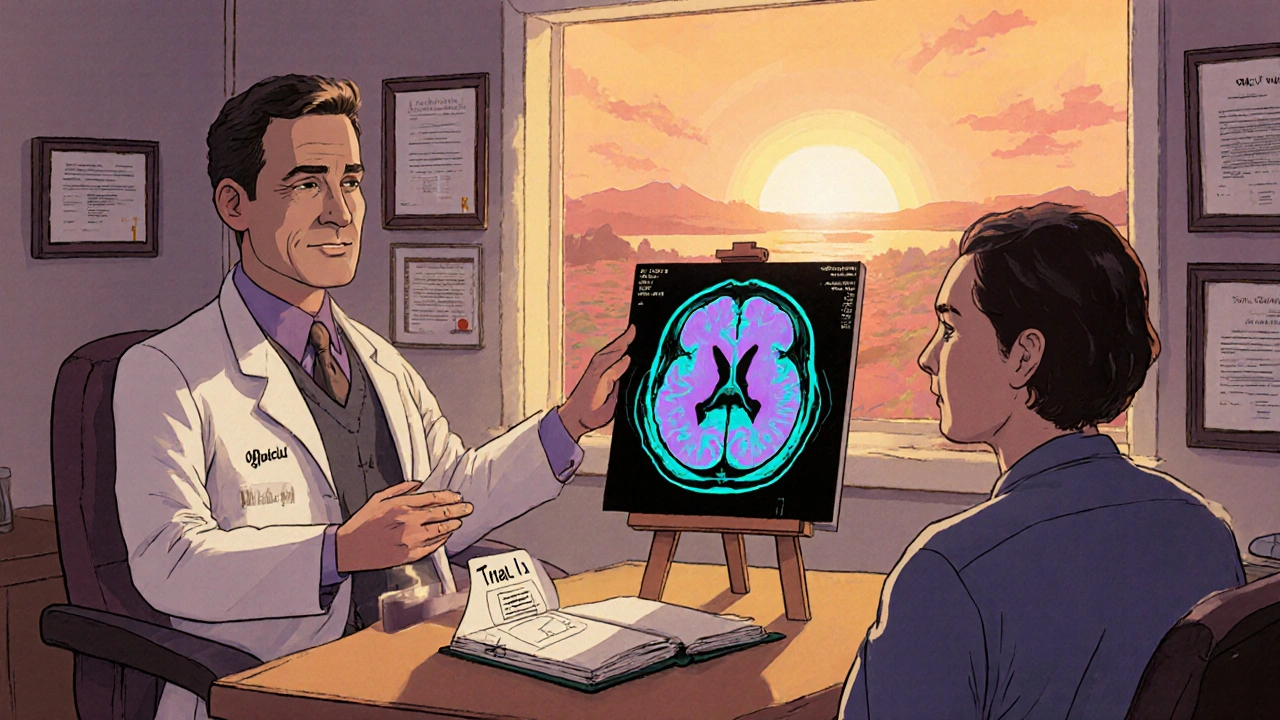
- Phase I/II (NCT05831245): Evaluates safety of cycloserine combined with radiotherapy in recurrent glioblastoma. Primary endpoint: dose‑limiting toxicity; secondary: progression‑free survival.
- Phase Ib (NCT05784219): Tests cycloserine plus pembrolizumab in metastatic triple‑negative breast cancer. Rationale: cycloserine may modulate the tumor microenvironment to improve checkpoint inhibition.
- Phase II (NCT05910477): Single‑agent cycloserine in patients with refractory pancreatic cancer. Primary outcome: objective response rate.
Results are still pending, but the fact that investigators are willing to move forward into combination arms signals confidence in the drug’s safety window.
Safety, Dosing, and Practical Considerations
Cycloserine’s most concerning adverse effect is neuropsychiatric toxicity. In tuberculosis treatment, rates of severe depression hover around 2 % at 500 mg daily. Oncology trials plan to start at lower doses (250 mg QD) and titrate upward while monitoring mood scales. Co‑administration with antidepressants appears safe, but clinicians must watch for serotonin syndrome if combined with SSRIs.
Renal clearance is the primary elimination route, so dose adjustments are needed for eGFR < 30 mL/min. Cycloserine also has a half‑life of 10 hours, making once‑ or twice‑daily dosing feasible.
Comparison with Other Repurposed Antibiotics
| Drug | Original Indication | Key Anticancer Target | Clinical Status (2025) |
|---|---|---|---|
| Cycloserine | Multi‑drug‑resistant TB | Serine racemase / folate metabolism | Phase I/II trials (glioblastoma, breast, pancreas) |
| Doxycycline | Bacterial infections | MMP inhibition / mitochondrial biogenesis | Phase II (metastatic prostate, ovarian) |
| Thioridazine | Psychosis | PI3K‑AKT pathway | Phase I (leukemia, solid tumors) |
| Linezolid | Gram‑positive infections | Protein synthesis in mitochondria | Pre‑clinical only |
Compared with doxycycline or thioridazine, cycloserine offers a distinct metabolic angle and a well‑characterized safety database, making it an attractive candidate for combination strategies.
Challenges and Open Questions
Despite promising signals, several obstacles remain:
- Blood‑brain barrier penetration: While cycloserine does cross the BBB, achieving therapeutic concentrations in deep brain lesions may require higher dosing or novel delivery systems.
- Biomarker identification: Not all tumors will respond. Early work suggests that high expression of serine racemase or folate‑cycle enzymes could predict sensitivity.
- Regulatory pathways: Repurposed drugs often fall into a gray area between new indications and off‑label use. Clear guidance from FDA/EMA would accelerate trial design.
- Intellectual property: Generic status of cycloserine limits commercial incentives, which could affect funding for larger Phase III studies.
Future Directions and What Patients Can Expect
If ongoing trials confirm safety and modest efficacy, cycloserine could become part of multi‑modal regimens, especially for tumors that are hard to treat with standard chemotherapy. Researchers are also exploring nanoparticle encapsulation to boost delivery to brain tissue and reduce systemic side effects.
For patients, the timeline means that cycloserine‑based regimens may appear in academic centers within the next 3‑5 years. Advocacy groups are already lobbying for expanded compassionate‑use programs, which could give access to patients with few alternatives.
Quick Summary
cycloserine cancer therapy is moving from the lab bench toward real‑world oncology trials. Its dual impact on NMDA signaling and folate metabolism gives it a unique edge among repurposed antibiotics. While neurotoxicity and biomarker selection are hurdles, early data suggest it could enhance existing treatments, particularly in glioblastoma and triple‑negative breast cancer. Keep an eye on the upcoming Phase I/II results - they’ll shape whether this old anti‑TB pill becomes a new weapon against cancer.
What makes cycloserine a candidate for cancer treatment?
Cycloserine blocks serine racemase and disrupts folate‑dependent nucleotide synthesis, two pathways that many tumors depend on for growth. Pre‑clinical models have shown tumor shrinkage and synergy with standard chemotherapy.
Is cycloserine already approved for cancer?
Not yet. It is still an investigational agent for oncology, with Phase I/II trials ongoing for glioblastoma, breast, and pancreatic cancers.
What are the main side effects to watch for?
Neuropsychiatric effects such as dizziness, anxiety, and rare depression are most concerning. Regular mood assessments and dose adjustments help manage risk.
Can cycloserine be combined with immunotherapy?
Early trials are testing cycloserine with pembrolizumab in triple‑negative breast cancer. The hypothesis is that metabolic disruption may make tumors more visible to immune cells.
How does cycloserine compare to other repurposed antibiotics?
Unlike doxycycline (which inhibits MMPs) or thioridazine (targets PI3K‑AKT), cycloserine works on serine metabolism and folate pathways. This gives it a complementary profile that may be useful in combination regimens.
When might patients see cycloserine as a treatment option?
If Phase II data are positive, larger trials could start by 2027, with potential compassionate‑use access in specialist centers a few years earlier.




Jacqueline Galvan
Thank you for sharing this thorough overview of cycloserine’s potential oncologic applications. I am particularly impressed by the detailed discussion of serine racemase inhibition and its downstream effects on NMDA signaling. Could you elaborate on whether biomarker stratification has been incorporated into the ongoing trials? The integration of metabolic profiling would certainly enhance the translational impact of this work.
Tammy Watkins
The prospect of repurposing an established anti‑tubercular agent such as cyclosorine for malignant disease is both intellectually stimulating and clinically consequential. From a mechanistic standpoint, the dual interference with serine racemase and folate‑dependent one‑carbon metabolism addresses two pivotal vulnerabilities in proliferating neoplastic cells. Importantly, the pre‑clinical data demonstrating synergistic tumor shrinkage when combined with temozolomide in glioblastoma models provides a compelling rationale for combinatorial strategies. Equally noteworthy is the observed sensitization of triple‑negative breast cancer to doxorubicin via down‑regulation of MDR1, which could mitigate chemoresistance in a notoriously aggressive subtype. While the neuropsychiatric safety profile of cyclosorine remains a legitimate concern, the proposed dose‑escalation schema commencing at 250 mg daily appears judicious. Moreover, the concurrent monitoring of mood indices and the permissibility of adjunctive antidepressants reflect an ethically responsible trial design. The inclusion of a Phase Ib arm evaluating synergy with pembrolizumab is particularly forward‑looking, as metabolic modulation may indeed augment immunogenic cell death. Nevertheless, the blood–brain barrier penetrance of cyclosorine, although documented, warrants quantitative pharmacokinetic assessment to ensure therapeutically relevant concentrations within intracerebral lesions. Biomarker development, such as quantifying serine racemase expression or folate cycle enzyme activity, could stratify patients most likely to benefit and streamline future enrollment. From a regulatory perspective, the repurposing pathway leverages existing safety dossiers, yet the paucity of intellectual property incentives may impede large‑scale commercial investment. In this context, public‑private partnerships and academic consortia could play a pivotal role in advancing Phase III trials. The potential to integrate nanoparticle encapsulation techniques to enhance central nervous system delivery also aligns with cutting‑edge drug‑delivery research. Clinicians should remain vigilant for rare but severe psychiatric adverse events, particularly in populations with prior mood disorders. Overall, the convergence of metabolic inhibition, chemosensitization, and immunomodulation positions cyclosorine as a uniquely multifaceted candidate in the oncology armamentarium. I eagerly anticipate the forthcoming trial results, which will undoubtedly clarify whether this venerable antibiotic can be resurrected as a bona‑fide anticancer therapy.
Dawn Bengel
Patriotism doesn't excuse sloppy drug hype. 😒
junior garcia
I get why patients are hopeful about any new avenue. The science sounds solid, yet we must stay grounded. I’m cheering for careful progress.
Dason Avery
Imagine a world where a decades‑old TB pill becomes a beacon of hope for cancer patients; it feels almost poetic. The intertwining of metabolism and immunity reminds us that biology is a tapestry of interconnected pathways. While we must tread cautiously, optimism fuels the relentless pursuit of better treatments. Let’s keep our minds open and our hearts resilient 😊
Casey Morris
Indeed, the data, while intriguing, , , suggest a nuanced interplay , between metabolic inhibition and tumor viability , that warrants further investigation , before broader clinical adoption.
Teya Arisa
It is commendable that the research community is exploring innovative repurposing strategies; such endeavors epitomize translational ingenuity. I wholeheartedly endorse continued rigorous evaluation of cyclosorine’s oncologic potential, and I remain optimistic about forthcoming findings. 🌟
Kester Strahan
From a pharmacodynamiic perspective, cyclosorine's inhibition of D‑serine synthesis modulates NMDA‑mediated proliferative signaling, which is particularly relevnt in glioma phenotypes. Moreover, the drug’s impact on folate‑one‑carbon cycles could precipitate synthetic lethality when paired with antimetabolites. However, the PK/PD profile in solid tumours remains under-characterized, necessitating robust pre‑clinical validation.
Doreen Collins
Supportive coaching matters.
In the broader context of drug repurposing, it is essential to recognize that while the allure of rapid clinical translation is compelling, the scientific community must not sacrifice methodological rigor for expediency; each phase of investigation-from in vitro assays to murine xenograft validation-provides critical data points that collectively shape our understanding of therapeutic viability and safety, and overlooking any of these stages could ultimately undermine patient trust and the translational pipeline.
HILDA GONZALEZ SARAVIA
I appreciate the comprehensive synthesis of cyclosorine’s mechanisms and the clear articulation of ongoing trial designs. Could we anticipate that future studies will incorporate metabolic imaging biomarkers to better quantify target engagement? This would certainly strengthen the mechanistic link between drug action and clinical response.
Amanda Vallery
Actually the cyclosorine data are more promising than many reserches think.
Carla Taylor
Sounds cool and hopeful. love seeing old drugs get a new spin
Kathryn Rude
Another repurposing fad? The science seems weak yet everyone jumps on the bandwagon 😑
Lindy Hadebe
The concept has merit but the evidence remains limited.