When you’re running a manufacturing line that produces medical devices, pharmaceuticals, or even high-precision aerospace parts, a single inaccurate measurement can mean the difference between a safe product and a life-threatening failure. Equipment calibration isn’t just paperwork-it’s the backbone of quality control. And validation? That’s how you prove the whole system works as intended, day in and day out. Skip either, and you’re not just risking non-compliance-you’re risking patient safety, regulatory fines, and lost trust.
What’s the Difference Between Calibration and Validation?
People mix these up all the time. Calibration checks if your instrument reads correctly against a known standard. If your digital scale says 100 grams when you put a 100-gram weight on it, it’s calibrated. Simple.
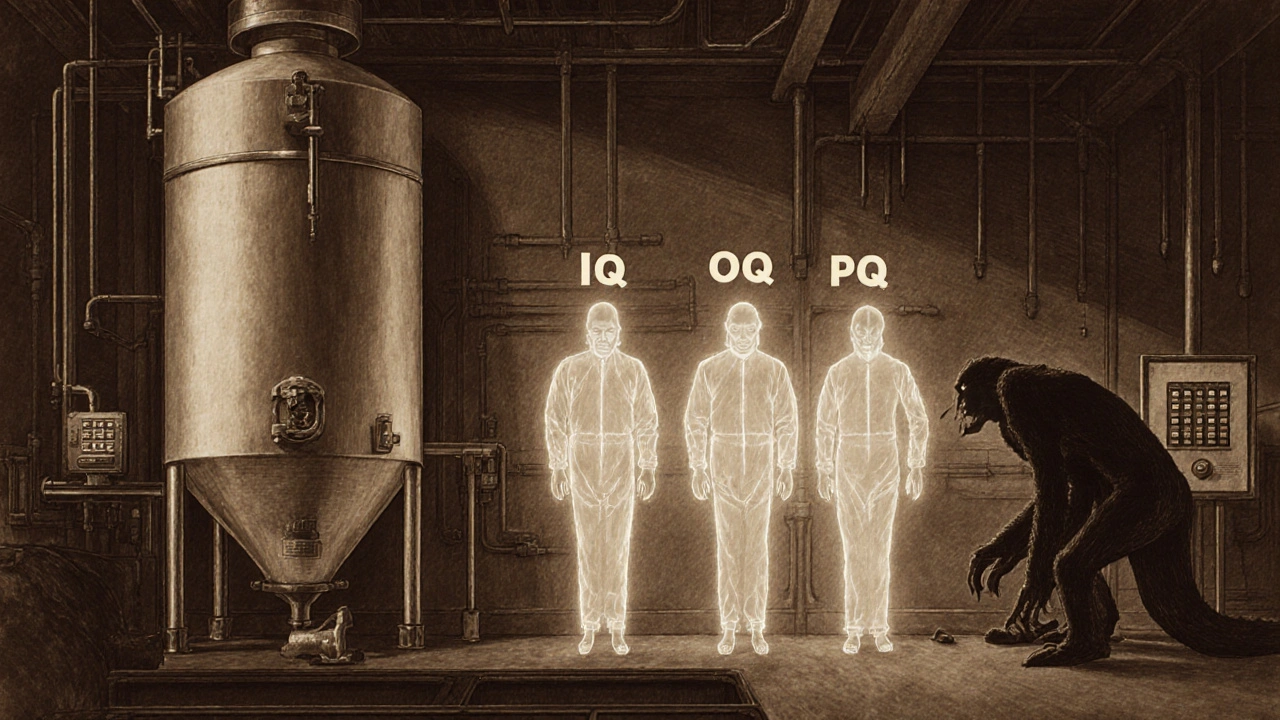
Validation goes deeper. It asks: Does this whole process do what it’s supposed to do? For example, a sterilizer might be perfectly calibrated to hit 121°C-but validation confirms that every item inside actually reaches that temperature long enough to kill all microbes. That’s Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). No calibration alone can do that.
Think of it this way: Calibration ensures your thermometer is accurate. Validation ensures the entire oven cooks the product safely every single time.
Regulatory Requirements: ISO 13485, FDA, and Beyond
If you’re in medical device manufacturing, ISO 13485:2016 is your bible. Clause 7.6 says you must calibrate equipment at specified intervals-or before use-and the standards you use must trace back to the International System of Units (SI). That means your 100-gram weight? It must be traceable to a national lab like NIST (in the U.S.) or NMI (in Australia), with documented uncertainty values.
The FDA’s 21 CFR Part 820 adds teeth to this. Records must be kept for the product’s lifetime plus two years. And if you’re using software to control a production line? Part 11 requires validation of that software too-algorithm drift counts as a failure.
CLIA rules for clinical labs are different. For high-complexity tests, you need calibration verification every six months or per manufacturer instructions-whichever is more frequent. And you must test three control levels daily. No exceptions. That’s because a wrong glucose reading can lead to insulin overdoses.
And don’t forget the EU MDR 2017/745. It demands traceability to BIPM standards, while the FDA accepts NIST. If you sell globally, you’re maintaining two parallel systems. That’s not optional-it’s a cost of doing business.
Calibration Intervals: One Size Doesn’t Fit All
Manufacturers often default to “calibrate every three months.” That’s safe-but wasteful. ISO 9001:2015 lets you set intervals based on risk. And smart companies do.
Here’s how it works:
- High-precision micrometers in aerospace? Calibrate every 3 months. These tools are used daily, handle tight tolerances, and any drift means scrapped parts.
- A basic lab thermometer in a food plant? Maybe once a year. It’s stable, rarely moved, and used in low-risk environments.
- Glucose meters in a clinic? Daily verification, not full calibration. They’re designed to be user-checked with control solutions.
- Nuclear density gauges on a highway site? Quarterly. Radiation sources decay. You can’t assume stability.
Dr. James Westad, an ASQ Fellow, calls out the mistake: “Calibrating stable equipment monthly while ignoring temperature swings? That’s not compliance-it’s noise.” One medical device maker in Melbourne cut calibration frequency by 40% by installing IoT sensors that monitored real-time environmental conditions. If the lab hit 25°C for 3 hours straight, the system flagged the equipment for re-check. No more calendar-based guesses.
Environmental Factors: The Silent Killer of Accuracy
Calibration isn’t done in a vacuum. Literally.
NIST Technical Note 1900 found that 57.8% of out-of-tolerance events happen when temperature swings exceed ±5°C from calibration conditions. Humidity? Even worse. In semiconductor manufacturing, 41.3% of calibration failures come from uncontrolled humidity-something SEMI E177-0323 explicitly warns about.
That pH meter your lab calibrated in a climate-controlled room? If it’s used in a humid cleanroom where humidity hits 80%, the readings drift. Fast. You need to either:
- Calibrate under the same conditions it’s used in
- Control the environment (ISO Class 5 chambers cost $85K-$120K)
- Adjust your calibration intervals based on real usage data
One biomedical engineer in Sydney extended her electronic scale calibration from quarterly to biannually after 18 months of stable data. Saved $18,500 a year. No audit issues. Just smart risk management.
The Cost of Getting It Wrong
GageList’s 2023 analysis of FDA warning letters showed 37.2% cited inadequate calibration. That’s not a footnote-it’s a red flag. A single violation can trigger a 483 observation, a product recall, or worse: a mandatory shutdown.
Small manufacturers (<50 employees) pay 22.3% more per device to stay compliant. Why? They can’t bulk-negotiate with calibration labs. They don’t have dedicated metrology staff. They’re doing it all manually.
And the hidden cost? Time. A 2024 FDA Small Business Survey found small manufacturers spend 15.2 hours a week just managing calibration records. Paper logs, Excel sheets, chasing signatures. That’s 60+ hours a month someone could be using to improve the process.
Digital Tools Are Changing the Game
Cloud-based calibration software like GageList and Trescal are no longer luxuries-they’re necessities.
Companies using these tools cut audit prep time by 63.2%. How? Automated reminders, digital certificates, real-time dashboards, and seamless integration with ERP systems. One Melbourne medical device firm went from 84 hours of audit prep per week to 31. That’s 53 hours back for engineers, QA staff, and managers.
But here’s the catch: 32.7% of negative reviews on G2 Crowd cite integration problems with legacy systems like SAP ECC 6.0. If your ERP is stuck in 2010, you’ll struggle. Don’t try to patch it. Plan for migration.
And now, the FDA’s 2024 Calibration Modernization Initiative requires all Class II and III device manufacturers to use electronic records by December 31, 2026. Paper is officially obsolete.
Validation: More Than Just a Checklist
Validation isn’t a one-time event. It’s a process.
- IQ: Did you install the equipment right? Are all parts present? Is the environment correct?
- OQ: Does it run within specs? Can it hit all its settings? Does it alarm when something’s wrong?
- PQ: Does it produce good product under real conditions? Run 10 batches. Test the output. Prove it.
For complex systems-like a robotic arm that welds implants-validation can cost $250,000 to $500,000 and take 18-24 months. But skipping it? That’s how you get a Class I recall. The FDA doesn’t care how much you spent on calibration. They care if your final product is safe.
And here’s the new frontier: ISO 13485:2016 Amendment 1 (March 2024) now requires calibration of AI/ML systems. Not just the sensors. The algorithm itself. If your AI predicts defect rates based on vibration data, you must validate that it doesn’t drift over time. Continuous monitoring is now mandatory.
What You Need to Do Right Now
Start here:
- Inventory every piece of measurement equipment. Give each one a unique ID.
- Classify them by risk: high, medium, low. Use the SAE AS9100D ‘Method 5’ approach-combine manufacturer guidance, historical data, and usage conditions.
- Map traceability: Can you prove each calibrator traces back to SI units? If not, fix it.
- Set intervals based on real data, not guesswork. Use condition-based triggers where possible.
- Automate records. Ditch the binders. Go digital.
- Validate critical equipment using IQ/OQ/PQ. Don’t skip PQ-it’s where most failures happen.
And train your team. ASQ’s Certified Calibration Technician (CCT) credential is held by over 14,000 professionals globally. Those with it earn 22.5% more. Invest in people. They’re your best defense against error.
What’s Coming Next
NIST is developing quantum-based calibration standards that will make electrical measurements 100x more accurate by 2030. That means you might only need to calibrate your high-end sensors once every two years instead of annually.
But here’s the bottleneck: 83.6% of calibration labs report technician shortages. In 2023, 47 accredited labs shut down because they couldn’t hire qualified people. If you’re outsourcing, your lead time is getting longer. Start building internal expertise now.
By 2027, 62.3% of quality leaders plan to integrate IoT sensors directly into equipment for continuous verification. Calibration won’t be an event-it’ll be a stream of data. The future isn’t about checking boxes. It’s about knowing, in real time, that your process is always in control.
How often should I calibrate my equipment?
There’s no universal rule. Calibration intervals depend on equipment type, usage, environment, and risk. High-precision tools like micrometers may need quarterly checks, while stable thermometers might only need annual calibration. Use historical performance data and environmental monitoring to set smart intervals-not just manufacturer recommendations.
What’s the difference between calibration and validation?
Calibration ensures your instrument reads accurately against a known standard. Validation confirms the entire system performs correctly for its intended use. For example, a scale might be calibrated to 100g, but validation proves the entire filling line consistently dispenses the right dose under real production conditions.
Is calibration mandatory for all equipment?
Yes, if the equipment affects product quality, safety, or regulatory compliance. ISO 13485 and FDA 21 CFR Part 820 require calibration for all measurement and test equipment used in quality control. Non-critical tools, like a basic ruler used only for rough layout, may be exempt-but document your rationale.
Can I use a manufacturer’s calibration certificate?
Only if it’s traceable to SI units and includes measurement uncertainty. Many manufacturers provide certificates, but they’re not always compliant. Check for NIST or BIPM traceability, environmental conditions during calibration, and the certificate’s validity date. If it’s missing any of these, you still need to verify it internally.
What happens if I don’t calibrate my equipment?
You risk non-conforming products, regulatory actions like FDA 483 notices or warning letters, product recalls, and reputational damage. GageList’s 2023 analysis found 37.2% of FDA warning letters cited inadequate calibration. In medical manufacturing, this can directly impact patient safety, leading to lawsuits or shutdowns.
Do I need to validate software used in manufacturing?
Yes. If software controls a process, monitors a parameter, or affects product quality, it must be validated under FDA 21 CFR Part 11 and ISO 13485. This includes AI systems that predict defects or adjust machine settings. Validation must include testing for algorithm drift and ongoing monitoring.
How can I reduce calibration costs without compromising compliance?
Use risk-based scheduling: extend intervals for stable equipment using historical data. Install IoT sensors to monitor environmental conditions and trigger recalibration only when needed. Automate recordkeeping with cloud-based software to cut administrative time. And train staff to spot early signs of drift-prevention is cheaper than correction.






vinod mali
Calibration isn't about checking boxes it's about keeping people safe
Jennie Zhu
Per ISO 13485:2016 Clause 7.6, traceability to SI units is non-negotiable. Furthermore, the documented measurement uncertainty must be quantified and maintained within the bounds of the equipment's tolerance specifications, as stipulated by the manufacturer's calibration certificate and the accredited laboratory's scope of accreditation.
Kathy Grant
I've seen labs spend $50k a year calibrating thermometers that never leave the same room, while the real problem is humidity swings no one's monitoring. It's not about following the calendar-it's about listening to the machine. One engineer I know started logging real-time environmental data and cut her calibration costs by 40% without a single audit finding. That's not luck-that's wisdom. We treat equipment like it's a clock that needs winding, but it's more like a plant-it tells you when it needs water if you pay attention.
Robert Merril
people think calibration is a chore but really its just the cost of not being an idiot
if your scale says 100g and its not 100g you got bigger problems than paperwork
also software validation is a nightmare i once saw a python script get flagged for drift because someone changed a decimal place in a config file and the FDA lost their mind
Noel Molina Mattinez
you think this is bad wait till you find out the calibration certs are fake and the NIST traceability is just a PDF someone downloaded from a forum
big pharma knows this and they dont care because theyre too busy paying off auditors
Roberta Colombin
It's important to remember that every piece of equipment we calibrate is part of a larger system that affects human lives. Taking the time to do it right, even when it feels tedious, is a quiet act of care. We don't always get to see the impact, but it's there-in every safe medication, every accurate diagnosis, every child who gets the right dose.
Dave Feland
The entire calibration regime is a controlled illusion designed by regulatory bodies to extract compliance fees from manufacturers. NIST? A government puppet agency. BIPM? A globalist cabal. The real truth is that most instruments are inherently stable-their drift is negligible unless deliberately induced by environmental sabotage or corporate negligence. And yes, I've seen the audit logs. Someone is manipulating the data.
Ashley Unknown
They say calibration is about safety but I've seen people lose their jobs over a 0.5% drift in a humidity sensor while the CEO flew to Bali on the company jet
and don't get me started on the software validation-there's a whole underground of people who write fake validation reports using ChatGPT and they're getting paid six figures
and the auditors? They just want their lunch money and a Starbucks gift card
it's a circus and we're all clowns wearing lab coats
my friend got fired because her calibration log had a typo and now she's on disability because the stress gave her panic attacks
and no one even apologized
Georgia Green
One thing I learned the hard way: if you calibrate in a climate-controlled room but use the tool in a hot warehouse, you're not calibrated-you're delusional. We started putting sensors on our critical gear and now we only recalibrate when the data says to. Cut our costs by a third. No one noticed. That's the goal.
Christina Abellar
Smart calibration saves money and lives. Simple as that.